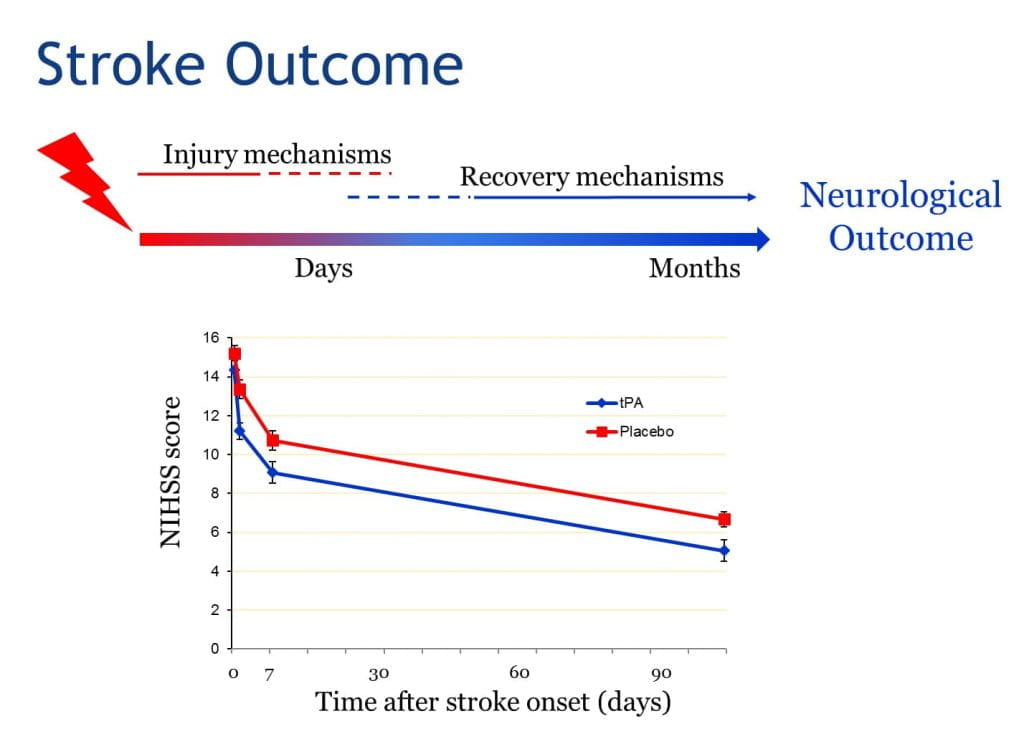
During the first hours after ischemic stroke onset, neurological deficits can be highly unstable – some patients spontaneously improve while others deteriorate. These early neurological changes are important because they have a large influence on long-term outcome. Potential mechanisms accounting for rapid improvement include fibrinolysis/ reperfusion, recruitment of collateral circulation, or endogenous neuroprotective mechanisms; while mechanisms leading to deterioration include thrombus propagation, peri-infarct spreading depression, or hemorrhagic transformation (HT). Tissue plasminogen activator (tPA), the only FDA-approved drug for the treatment of acute ischemic stroke (AIS), enhances the likelihood of fibrinolysis and reperfusion; but also increases the chances of HT. We hypothesize that genetic variants that affect pathogenic mechanism during acute ischemia may influence early neurological outcomes after AIS, and may also modulate response to IV tPA. In this grant, we propose to identify genetic variants associated with early neurological outcome in AIS patients (untreated or treated with IV tPA). These data will permit us to find novel genes/pathways and potential therapeutic targets that could improve outcome after AIS, and perhaps enhance tPA efficacy.
Publications
- Mola-Caminal M, Carrera C, Soriano-Tárraga C, Giralt-Steinhauer E, Díaz-Navarro RM, Tur S, Jiménez C, Medina-Dols A, Cullell N, Torres-Aguila NP, Muiño E, Rodríguez-Campello A, Ois A, Cuadrado-Godia E, Vivanco-Hidalgo RM, Hernandez-Guillamon M, Solé M, Delgado P, Bustamante A, García-Berrocoso T, Mendióroz M, Castellanos M, Serena J, Martí-Fàbregas J, Segura T, Serrano-Heras G, Obach V, Ribó M, Molina CA, Alvarez-Sabín J, Palomeras E, Freijo M, Font MA, Rosand J, Rost NS, Gallego-Fabrega C, Lee JM, Heitsch L, Ibanez L, Cruchaga C, Phuah CL, Lemmens R, Thijs V, Lindgren A, Maguire J, Rannikmae K, Sudlow CL, Jern C, Stanne TM, Lorentzen E, Muñoz-Narbona L, Dávalos A, López-Cancio E, Worrall BB, Woo D, Kittner SJ, Mitchell BD, Montaner J, Roquer J, Krupinski J, Estivill X, Rabionet R, Vives-Bauzá C, Fernández-Cadenas I, Jiménez-Conde J. PATJ Low Frequency Variants Are Associated With Worse Ischemic Stroke Functional Outcome. Circ Res. 2019;124(1):114-120.
- Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, Amouyel P, Ay H, Bartz TM, Benavente OR, Bevan S, Boncoraglio GB, Brown RD Jr, Butterworth AS, Carrera C, Carty CL, Chasman DI, Chen WM, Cole JW, Correa A, Cotlarciuc I, Cruchaga C, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018; 50(4):524-537. PMCID: PMC5968830
- Carrera C, Jiménez-Conde J, Derdak S, Rabionet K, Vives-Bauzá C, Soriano-Tárrega C, Giralt-Steinhauer E, Mola M, Diaz-Navarro RM, Tur S, Muiño E, Gallego-Fabrega C, Beltran S, Roquer J, Ruiz A, Sotolongo-Grau O, Krupinski J, Lee JM, Cruchaga C, Delgado P, Malik R, Worrall BB, Seshadri S, Montaner J, Fernández-Cadenas I; Metastroke Consortium, ISGC Consortium and Genestroke Consortium. Whole exome sequencing analysis reveals TRPV3 as a risk factor for cardioembolic Stroke. Thromb Haemost 2016; 8:116(6).
- Tosto G, Bird TD, Bennett DA, Boeve BF, Brickman AM, Cruchaga C, Faber K, Foroud TM, Farlow M, Goate AM, Graff-Radford NR, Lantigua R, Manly J, Ottman R, Rosenberg R, Schaid DJ, Schupf N, Stern Y, Sweet RA, Mayeux R; National Institute on Aging Late-Onset Alzheimer Disease/National Cell Repository for Alzheimer Disease (NIA-LOAD/NCRAD) Family Study Group. The Role of Cardiovascular Risk Factors and Stroke in Familial Alzheimer Disease. JAMA Neurol 2016; 73(10):1231-1237. PMCID: PMC5155512